
However, solution-phase synthesis of early transition metal chalcogenide nanocrystals is challenging due, in part, to their high crystallization energy and oxophilicity. Without exaggerating, we can say that our view of condensed matter has undergone two revolutions in the 20th century: first, the introduction of quantum physics in 1930, then the recognition of "self-similar" structures and the resulting scaling laws around 1970. These nanomaterials exhibit unique electronic and optical properties, allowing for a broad range of applications, depending on their chemical composition and crystal structure. 2) Our profound view of the mechanisms has evolved: in particular, the very universal properties of fluctuations near a critical point - described by Kadanoff's qualitative analysis and specified by an extraordinary theoretical tool: the renormalization group.
#Phase transitions how to#
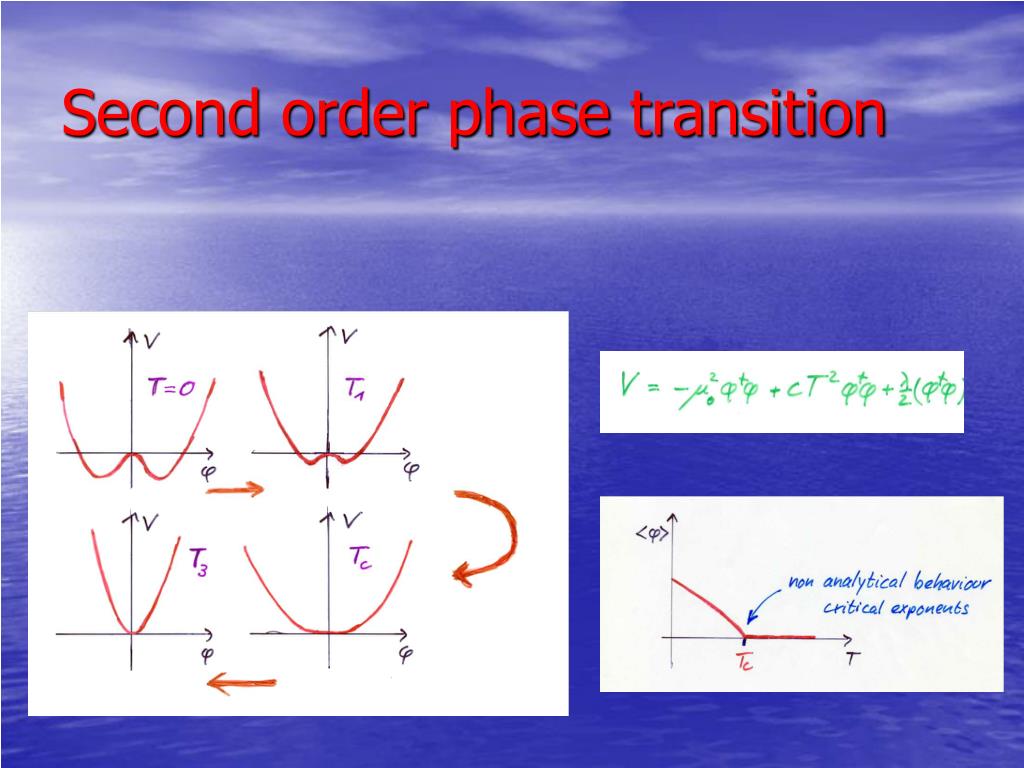
There is now a real zoology of transitions, but we know how to classify them based on Landau's superb idea. And other superfluids were recognized later: helium 4, helium 3, the matter constituting atomic nuclei and neutron stars. We review the effects of excited-state quantum phase transitions (ESQPTs) in interacting many-body systems with finite numbers of collective degrees of freedom. In addition, the extraordinary phenomenon of su perconductivity in certain metals appeared at the beginning of the 20th cen tury. Furthermore, phase transition between phases and was found at a pressure and temperature of 0.35 GPa and 400 K, respectively.

Then for magnetism, illustrated in France by Louis Neel, and ferro electricity. (physics) The transition between thermodynamic phases of a physical system, especially one between the solid, liquid, and gaseous phases of a substance. It has now grown enormously in two directions: 1) The transitions have multiplied: first between a solid and a solid, par ticularly for metallurgists. " The science of the states of matter was born in the 19th century.

We learned in school that matter exists in three forms: solid, liquid and gas, as well as other more subtle things such as the fact that "evaporation produces cold.


 0 kommentar(er)
0 kommentar(er)
